Answer: The solid form of carbon dioxide
In simple terms: Dry ice is the solid occurence of CO2 – also called carbon dioxide. The ‘dry’ part of the name comes from the fact that it skips the liquid phase when heating up and turns into gas instantly during a process called sublimation. The ‘ice’ part comes from the substance being incredibly cold with sublimation happening at -109.3 °F (equivalent to -78.5 °C) at normal pressure levels. This extreme cold provides for its most important usage cases too, like utilizing it as a cooling agent for food and other goods.

Dry ice definitely comes in handy when you need to keep stuff chilly, but it is actually most sought after during October, due to, of course Halloween. The misty-foggy effect it creates when sublimating is the perfect addition to any scary Halloween party or for another example, rock concerts.
So that is dry ice in brief. Now let’s take a look at what actually carbon dioxide is.
CO2
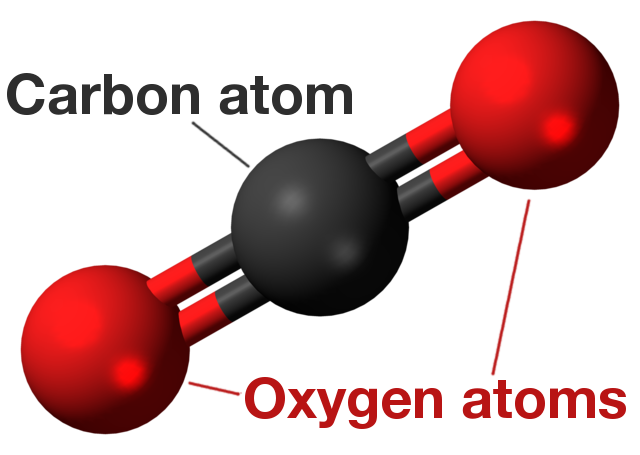
Carbon dioxide is a gas found naturally in the athmosphere of Earth (with a concentration of 0.04% compared to say oxygen’s 21%). It’s molecule consists of one carbon atom covalently attached to two oxygen atoms hence the chemical formula CO2.
It doesn’t have a liquid form under normal circumstances, only if pressure is sufficiently high (5.2 bar; 75 psi). This is the reason why dry ice turns into gas instantly at a certain temperature (-109.3 Degress Fahrenheit), skipping the usual solid-liquid-gas phase steps as seen with water for example.
We have to note here that CO2 is a greenhouse gas commonly identified as a major contributor to global warming.
So dry ice the solid form of this colorless gas, what’s next? Let’s look at some properties of dry ice.
Properties of Dry Ice
Here we list six quick facts about dry ice below that everyone should know:
- Colorless, just like it’s gas form.
- It’s unburnable (some fire extinguishers use pressurized carbon dioxide to put out fires)
- It’s very cold and can cause frostbite when touched without necessary safety precautions.
- It doesn’t conduct heat or electricity very well.
- It’s relatively easy to make in large quantities.
- Can be very toxic if stored or used incorrectly.